Verify™ Process Challenge Devices
Verify™ Process Challenge Devices
Effectively monitor steam and ethylene oxide gas sterilization processes with fully self-contained biological indicators
Product Benefits
Verify™ Process Challenge Devices are fully self-contained biological indicators (SCBIs) designed to effectively monitor steam and ethylene oxide (EO) gas sterilization processes. Each test pack includes a chemical indicator, a vial containing dual spore species and a specially formulated soybean casein digest growth medium. Manufactured under controlled conditions, they meet stringent quality requirements and provide a simple and reliable method to monitor sterilization processes.
- Enhanced Productivity: Eliminates the need for aseptic handling, streamlining sterilization monitoring processes.
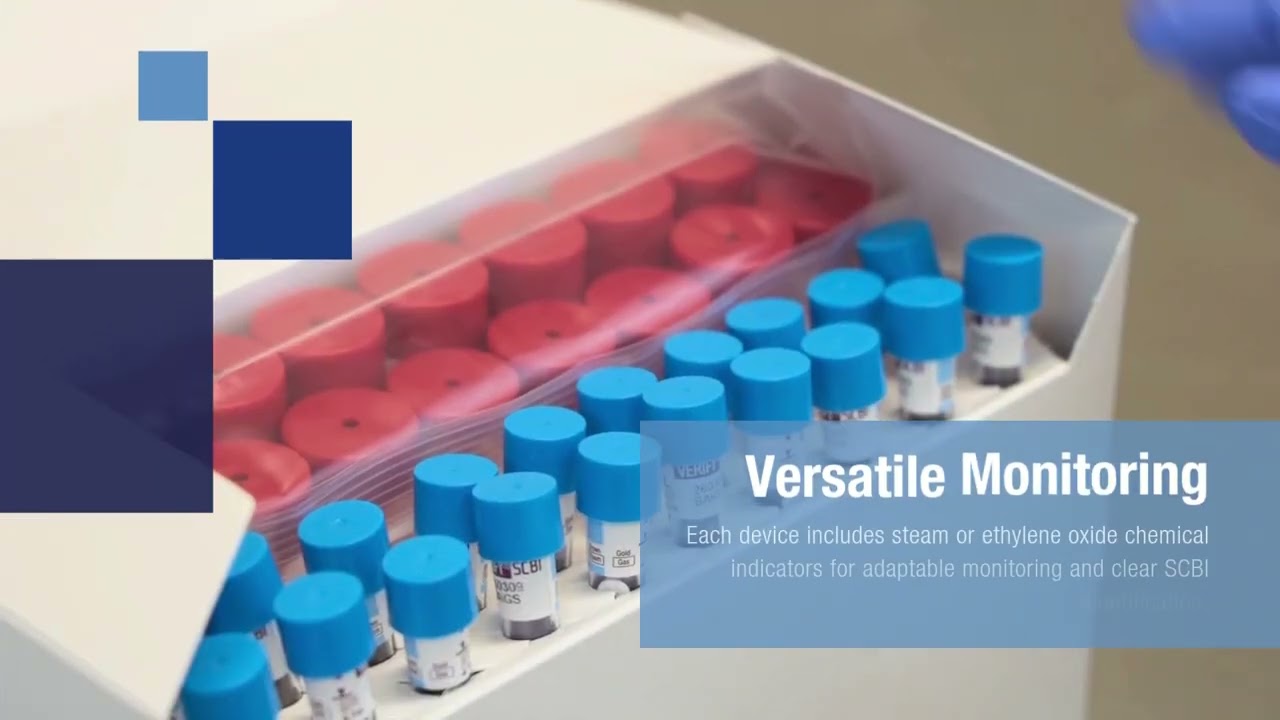
- Versatile Monitoring: Each process challenge device features steam or EO chemical indicators to provide adaptable monitoring and clear identification of processed SCBIs.
- Superior Reliability: Each process challenge device was developed to present a greater challenge to air removal and steam penetration compared to the Association for the Advancement of Medical Instrumentation (AAMI) test packs to optimize effectiveness in sterilization verification.
Downloadable Resources
Related Products
Certificate of Analysis
Enter Product ID and Lot Number Below
Safety Data Sheets (SDS)
Safety Data Sheets (SDS) provide information on STERIS products for purposes of health, safety and environmental requirements.
Join our email list for the latest industry news and product updates.
By clicking Subscribe you're confirming that you agree with our Terms and Conditions.







